
TGA approved indication for Utrogestan 200 mg, 300 mg and 400 mg (vaginal micronised progesterone)1:
Luteal phase support during assisted reproduction.1
Treatment of threatened miscarriage in women with a history of ≥ 3 previous miscarriages and women with < 3 miscarriages who have a reduced chance of future pregnancy. Benefit of treatment was greatest in women with ≥ 3 previous miscarriages.1
Prevention of preterm birth in women with singleton pregnancy who have a short cervix (midtrimester sonographic cervix ≤25 mm) and/or a history of spontaneous preterm birth.1
Wholesale order numbers
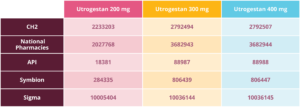 Available to order now. Please contact your preferred supplier directly for their order codes
Available to order now. Please contact your preferred supplier directly for their order codes
How to insert Utrogestan
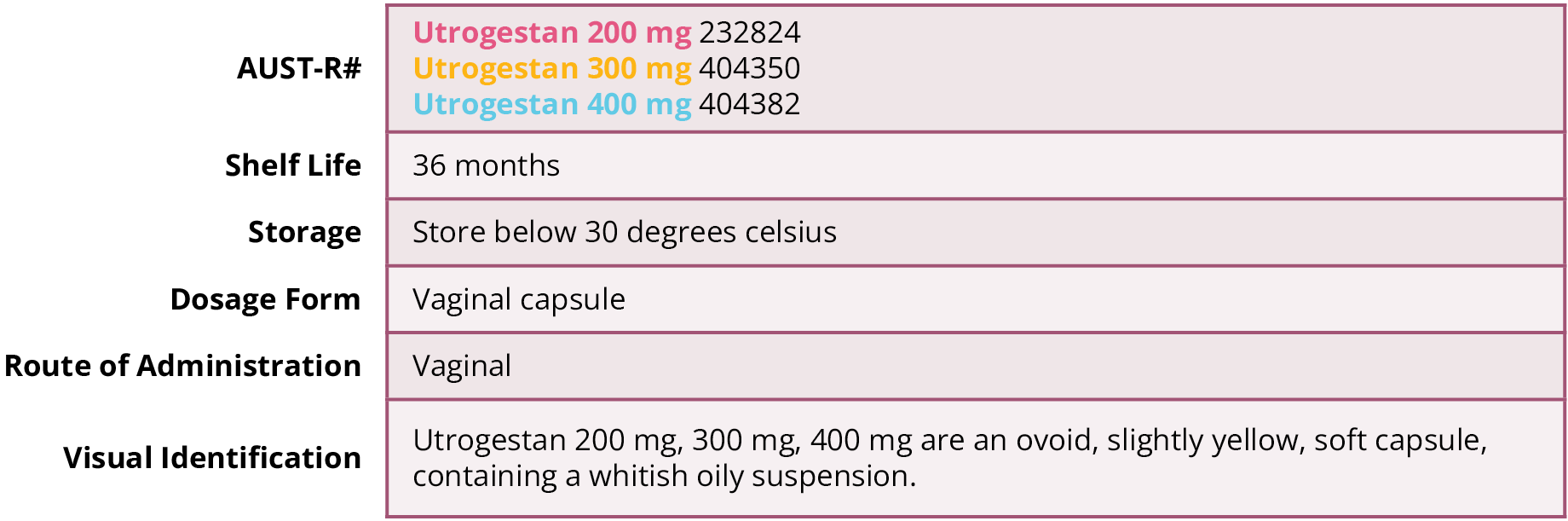
Pharmaceutical particulars
References: 1. Utrogestan (micronised progesterone) Product Information. 2. van der Linden et al. Coch Datab Syst Rev 2015, Issue 7. Art. No.: CD009154. 3. Child T et al. Reprod Med 2018;36:630–45. 4. Devall AJ et al. Cochrane Datab Syst Rev 2021, Issue 4. Art. No.: CD013792. 5. Care A et al. BMJ 2022;376: doi: https://doi.org/10.1136/bmj-2021-064547. 6. The EPPPIC Group. Lancet 2021;397:1183–94. 7. Romero R et al. Am J Obstet Gynaecol 2018;218(2):161-80.














