The efficacy of vaginal progesterone for preterm birth prevention in women with a short midtrimester cervix was initially reported in the New England Journal of Medicine in 2017. In this study, daily administration of Utrogestan 200 mg from 24 to 34 weeks of gestation significantly reduced the risk of preterm birth (<34 weeks) by 43% compared to placebo (RR 0.57; 95% CI 0.35–0.92; p=0.02).1,4
These findings were confirmed in a systematic review and meta-analysis of 974 women from 5 high-quality clinical trials.1-7 In women with a singleton pregnancy and a midtrimester short cervix (≤25 mm), vaginal progesterone significantly and consistently reduced the risk of preterm birth from <28 to <36 weeks of gestation, compared to placebo. The primary outcome – preterm birth <33 weeks of gestation – saw a significant 38% reduction in preterm birth with vaginal progesterone over placebo (RR 0.62, p=0.0006; see figure below).2
In addition, vaginal progesterone resulted in significant improvements in perinatal outcomes, including reduced risk of respiratory distress syndrome (RR 0.47, p=0.007), low birthweight <2500g (RR 0.82, p=0.03), NICU admission (RR 0.68, p=0.003) and neonatal morbidity and mortality (RR0.59, 0.02).2
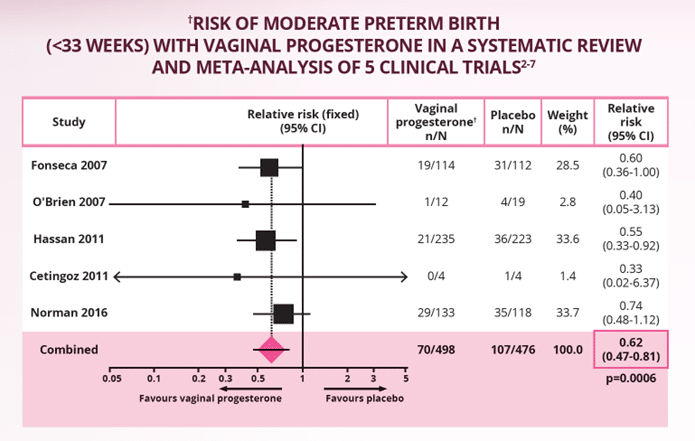 Adapted from Romero R et al. 2018.2
Adapted from Romero R et al. 2018.2
Note: Vaginal progesterone preparations included Utrogestan® micronised progesterone capsules,3,4 micronised progesterone gel,5,6 and a compounded progesterone suppository.7
RR, risk reduction. References: 1. Utrogestan (micronised progesterone) Product Information. Last updated 22 February 2022. 2. Romero R et al. Am J Obstet Gynaecol 2018;218(2):161-80. 3. Norman JE et al. Lancet 2016;387:2106-16. 4. Fonseca EB et al. N Engl J Med 2007;357:462-9. 5. O’Brien JM et al. Ultrasound Obstet Gynecol 2007;30:687-96. 6. Hassan SS et al. Ultrasound Obstet Gynecol 2011;38:18-31. 7. Cetingoz E et al. Arch Gynecol Obstet 2011;283:423-9.

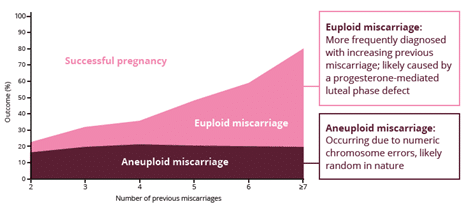
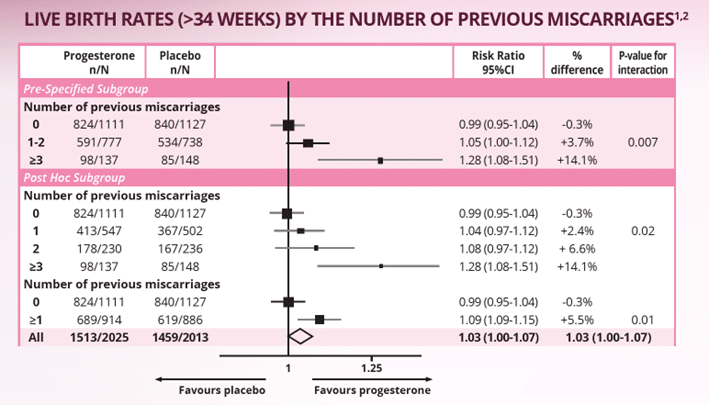
 Adapted from Romero R et al. 2018.2
Adapted from Romero R et al. 2018.2












